Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(4); 2023 > Article
-
Review
A stepwise approach to fine needle aspiration cytology of lymph nodes -
Yosep Chong,1
 , Gyeongsin Park1
, Gyeongsin Park1 , Hee Jeong Cha2
, Hee Jeong Cha2 , Hyun-Jung Kim3
, Hyun-Jung Kim3 , Chang Suk Kang4, Jamshid Abdul-Ghafar1
, Chang Suk Kang4, Jamshid Abdul-Ghafar1 , Seung-Sook Lee5
, Seung-Sook Lee5 -
Journal of Pathology and Translational Medicine 2023;57(4):196-207.
DOI: https://doi.org/10.4132/jptm.2023.06.12
Published online: July 11, 2023
1Department of Hospital Pathology, The Catholic University of Korea, College of Medicine, Seoul, Korea
2Department of Pathology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
3Department of Pathology, Inje University Sanggye Paik Hospital, Seoul, Korea
4Department of Pathology, Samkwang Medical Laboratories, Seoul, Korea
5Department of Pathology, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- Corresponding Author: Yosep Chong, MD, PhD, Department of Hospital Pathology, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 271 Cheonbo-ro, Uijeongbu 11765, Korea Tel: +82-32-820-3160, Fax: +82-32-820-3877, E-mail: 'ychong@catholic.ac.kr'
© 2023The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- CLINICAL FINDINGS
- LOW-POWER PATTERN OF LYMPH NODE FINE NEEDLE ASPIRATION CYTOLOGY
- HIGH-POWER PATTERN OF LYMPH NODE FINE NEEDLE ASPIRATION CYTOLOGY
- DISEASE-SPECIFIC DIAGNOSTIC CLUES
- SYDNEY CLASSIFICATION
- SUGGESTED DIAGNOSTIC ALGORITHM FOR LYMPH NODE FINE NEEDLE ASPIRATION CYTOLOGY
- CONCLUSION
- Supplementary Information
- Notes
- References
Abstract
- The cytological diagnosis of lymph node lesions is extremely challenging because of the diverse diseases that cause lymph node enlargement, including both benign and malignant or metastatic lymphoid lesions. Furthermore, the cytological findings of different lesions often resemble one another. A stepwise diagnostic approach is essential for a comprehensive diagnosis that combines: clinical findings, including age, sex, site, multiplicity, and ultrasonography findings; low-power reactive, metastatic, and lymphoma patterns; high-power population patterns, including two populations of continuous range, small monotonous pattern and large monotonous pattern; and disease-specific diagnostic clues including granulomas and lymphoglandular granules. It is also important to remember the histological features of each diagnostic category that are common in lymph node cytology and to compare them with cytological findings. It is also essential to identify a few categories of diagnostic pitfalls that often resemble lymphomas and easily lead to misdiagnosis, particularly in malignant small round cell tumors, poorly differentiated squamous cell carcinomas, and nasopharyngeal undifferentiated carcinoma. Herein, we review a stepwise approach for fine needle aspiration cytology of lymphoid diseases and suggest a diagnostic algorithm that uses this approach and the Sydney classification system.
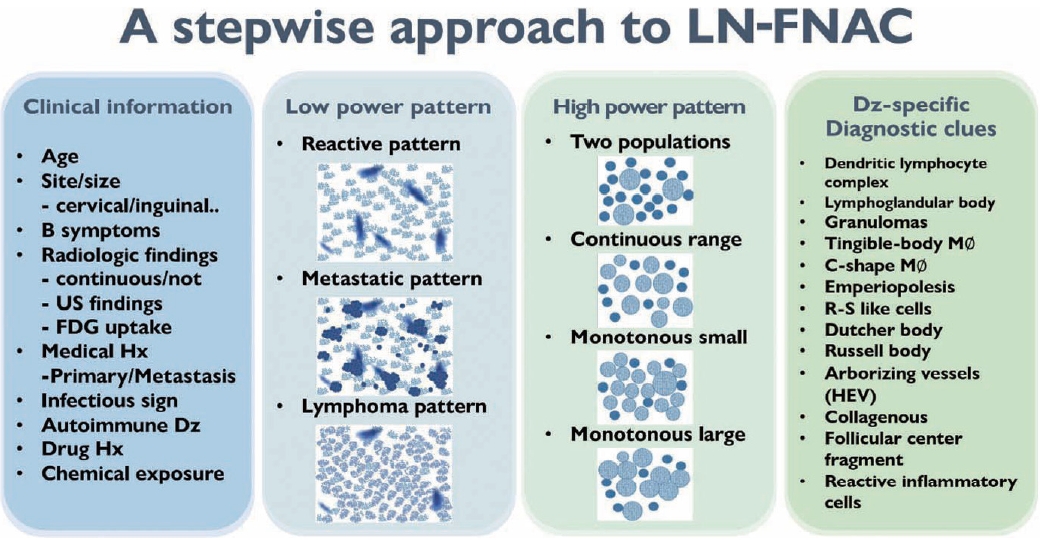
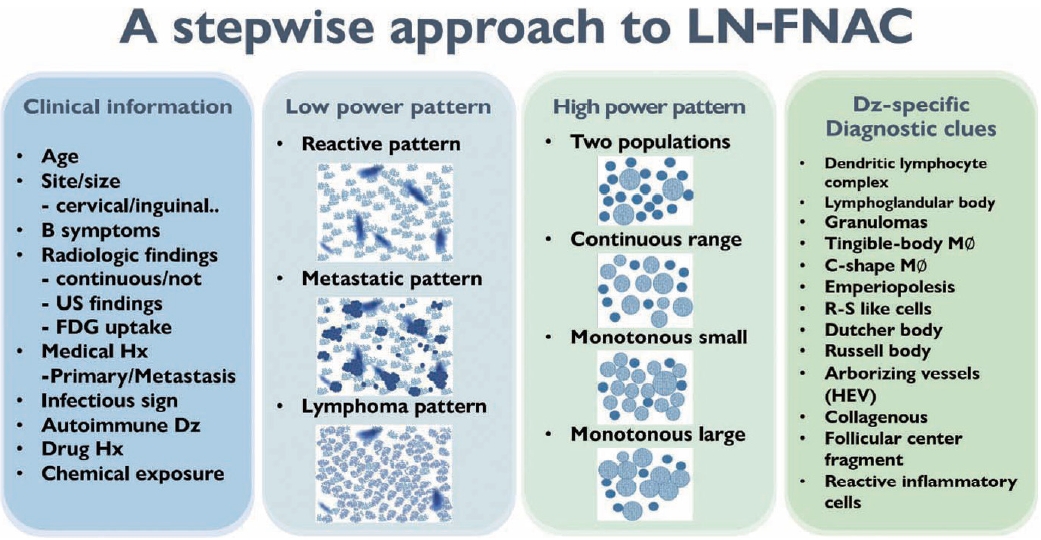
- Fig. 2 summarizes the stepwise approach to lymph node FNAC. Before cytological interpretation, it is important to review the clinical information. In order to formulate a proper differential diagnosis, the following clinical features are needed: age, site (cervical/inguinal), lymph node size, presence of B symptoms (fever, weight loss, night sweating), previous medical history (primary or metastatic), infectious signs, autoimmune disease, and history of drug or chemical exposure.
- Radiological findings, including mostly ultrasonographic and positron emission tomography computed tomography (PET/CT) findings, are helpful to narrow the differential diagnosis ( Supplementary Table S1, Supplementary Fig. S1). In benign lymphadenopathy, the lymph nodes are relatively small, oval-shaped, and hypoechoic with a regular well-demarcated border. The general architecture is preserved, with a visible hilum without obvious necrosis or calcification. In metastatic lesions, lymph nodes are usually large, round, and hypoechoic with irregular, ill-defined blurred borders that suggest capsular invasion. The architecture can be destroyed by metastatic tumors, leading to a heterogeneous internal structure without a visible hilum. Cortical nodules, cystic tumor necrosis, and calcifications can occur. In malignant lymphomas, lymph nodes are continuously enlarged/ conglomerated and hypoechoic with cortical thickening and no visible hilum. On PET/CT, metastatic lesions or malignant lymphomas demonstrate high fluorodeoxyglucose uptake more frequently than do normal lymph nodes.
- On microscopic examination, a comprehensive interpretation of low-power patterns, high-power patterns, and disease-specific diagnostic clues is essential for successful differential diagnosis.
CLINICAL FINDINGS
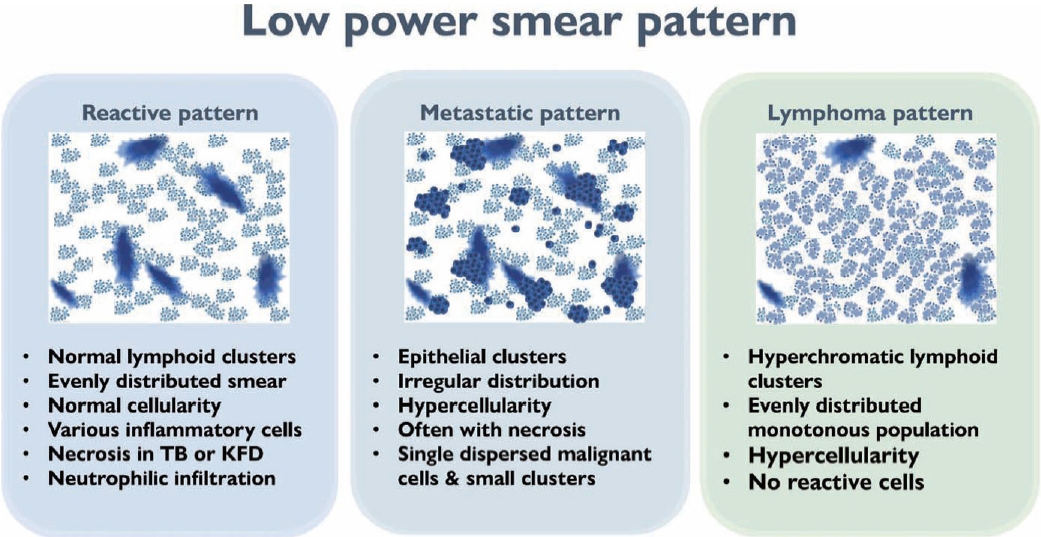
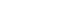
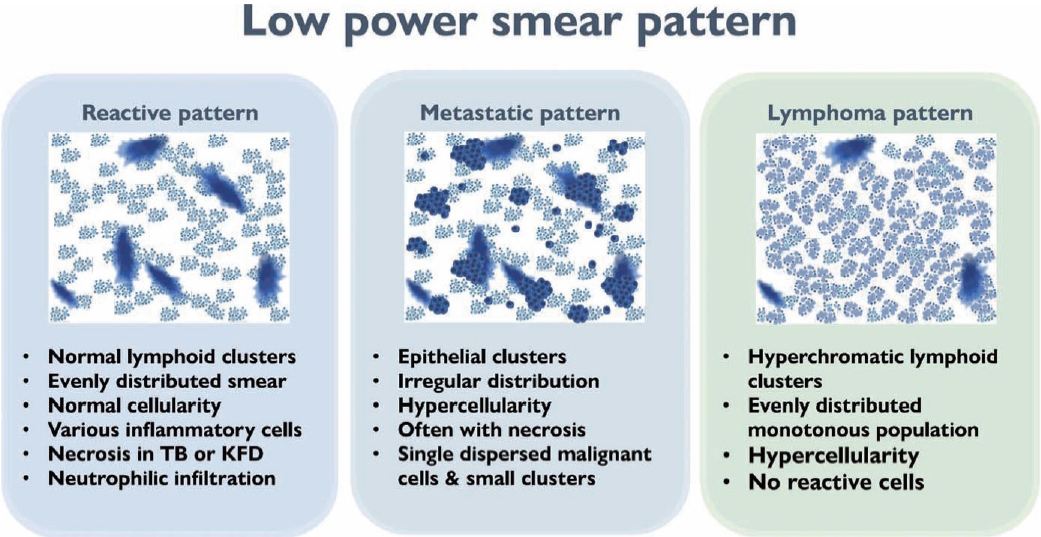
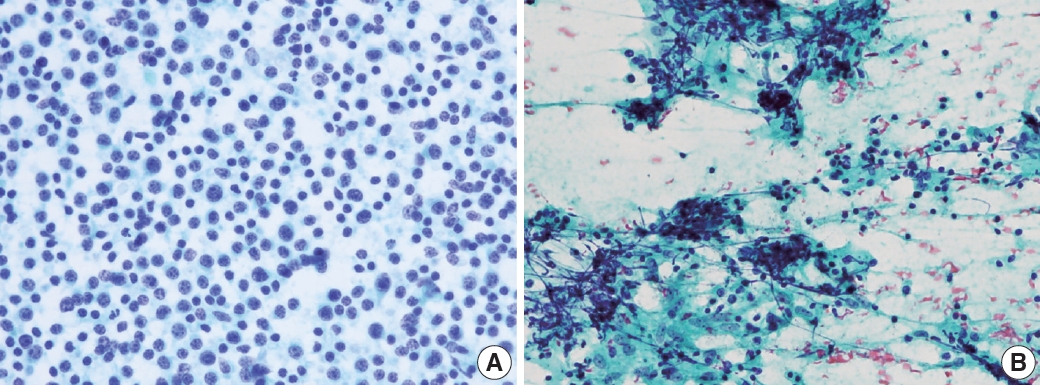
- The first step in lymph node FNAC is to assess the low-power magnification patterns. Lymphadenopathy can be broadly categorized into three patterns: reactive, metastatic, and lymphoma (Fig. 3). The reactive pattern comprises an evenly distributed smear with normal cellularity and some normal lymphoid clusters. Diverse inflammatory cells may be observed according to the type of lymphadenopathy. Necrosis or granulomas may be observed in tuberculosis or KFD. Neutrophilic smears suggest a benign condition.
- The metastatic pattern involves an irregularly distributed hypercellular smear with prominent epithelial clusters and is often accompanied by necrosis. Single dispersed malignant cells and small-to-large clusters are observed.
- The lymphoma pattern shows an evenly distributed hypercellular smear with monotonous hyperchromatic lymphoid clusters. Reactive inflammatory cells (such as neutrophils) are uncommon. Occasional multinucleated, large, atypical cells such as Reed-Sternberg cells and ill-defined granulomas with occasional eosinophils can be found in HL.
LOW-POWER PATTERN OF LYMPH NODE FINE NEEDLE ASPIRATION CYTOLOGY
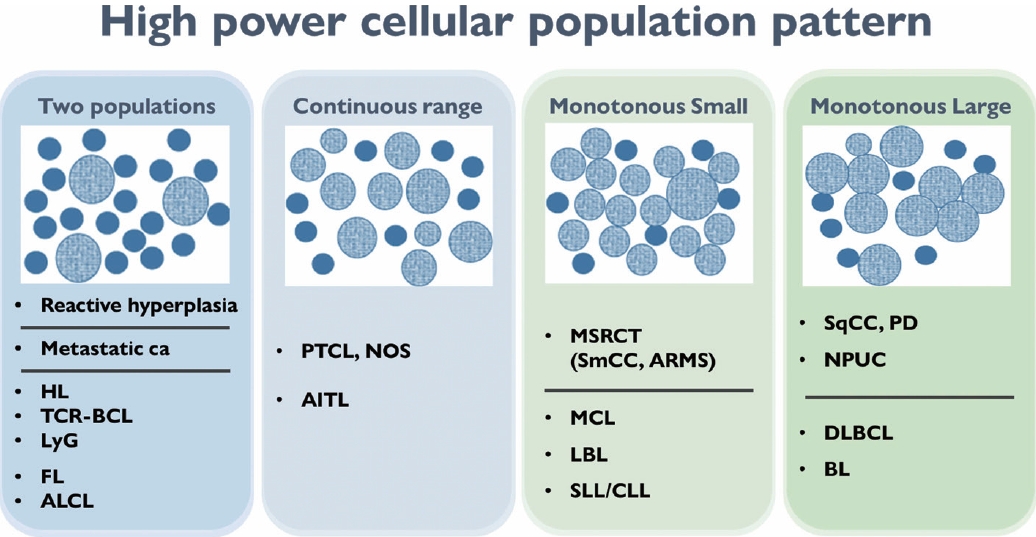
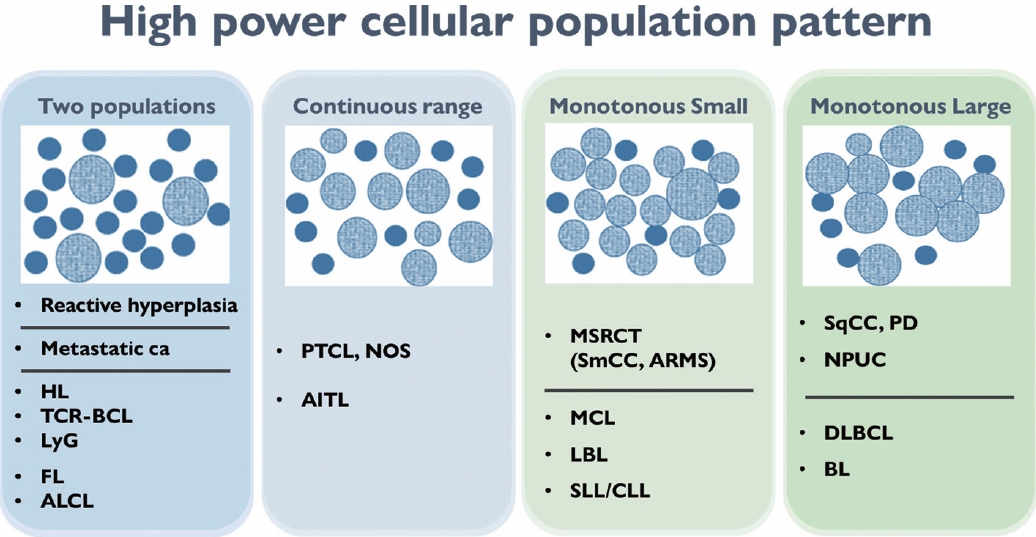
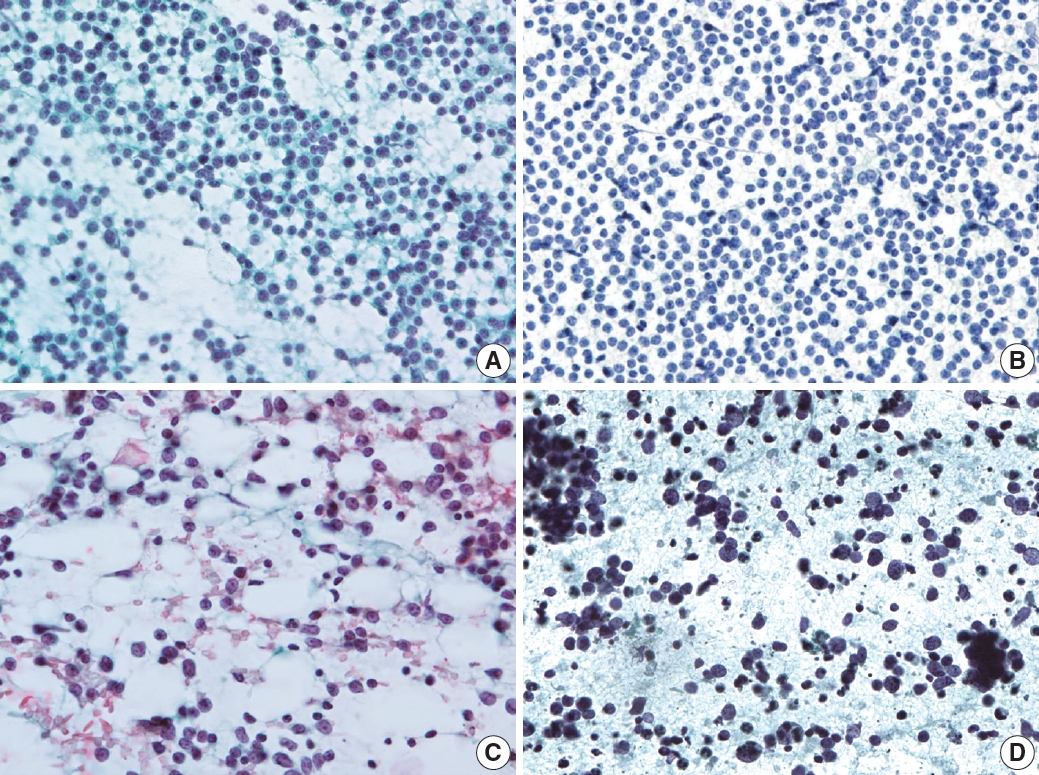
- The second step for lymph node FNAC is to assess high-power magnification cell population patterns. There are four broad patterns: two-cell, continuous range, monotonously small, and monotonously large population (Fig. 4). The two-cell population pattern is a smear pattern with two distinct cell populations. This pattern can be seen in reactive hyperplasia; metastatic carcinomas; and diverse lymphomas including HL, FL, and anaplastic large cell lymphoma. Rarely, T-cell/histiocyte-rich B-cell lymphoma (TCR-BCL) and lymphomatoid granulomatosis can also present with this cytological pattern [2].
- The second pattern is a smear with a continuous range of variably sized cells. In this pattern, there is an obvious dominant cell population of atypical lymphocytes and diverse inflammatory cells such as eosinophils. PTCL and angioimmunoblastic lymphoma, which are representative examples of this pattern, can present with variable inflammatory/reactive cells.
- The third pattern is a small cell population that predominantly presents as monotonously small, atypical lymphoma cells with few reactive cells. This category includes malignant small round cell tumors, such as metastatic small cell carcinoma or small round cell sarcoma, and low-grade B-cell lymphoma such as MCL or small lymphocytic lymphoma. However, malignant small round cell tumors generally have slightly larger nuclei than low-grade B-cell lymphomas.
- The fourth pattern is a large cell population of mostly monotonously large atypical malignant cells. For metastasis, poorly differentiated squamous cell carcinoma (such as basaloid type) and undifferentiated nasopharyngeal carcinoma are the major differential diagnoses. Diffuse large B-cell and Burkitt lymphoma are common examples of lymphoma.
- Two-cell population pattern
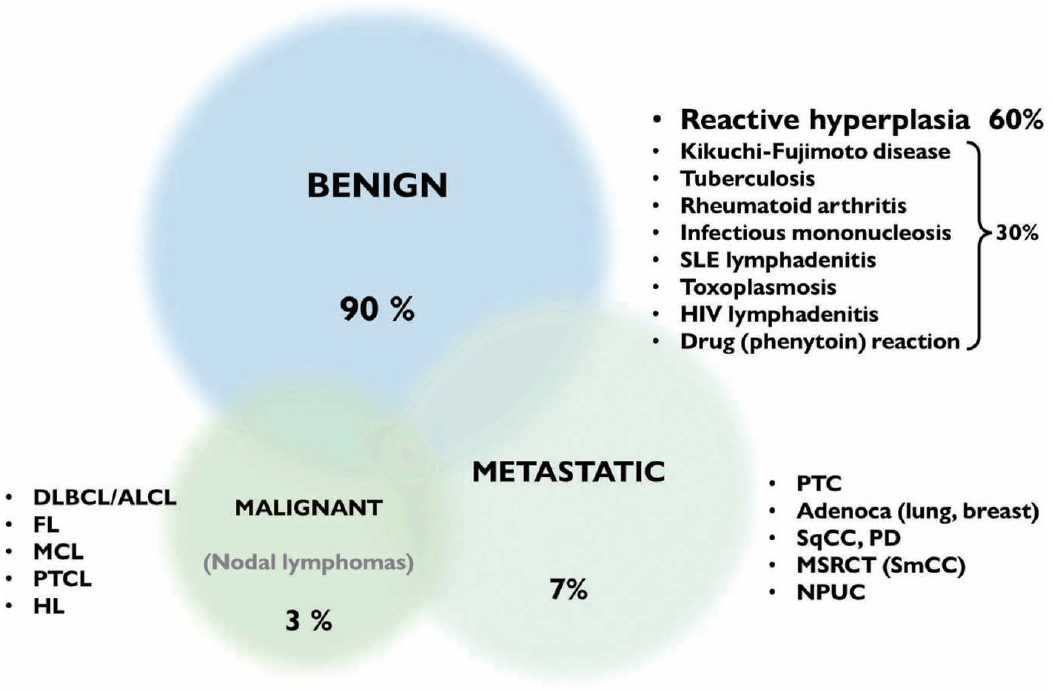
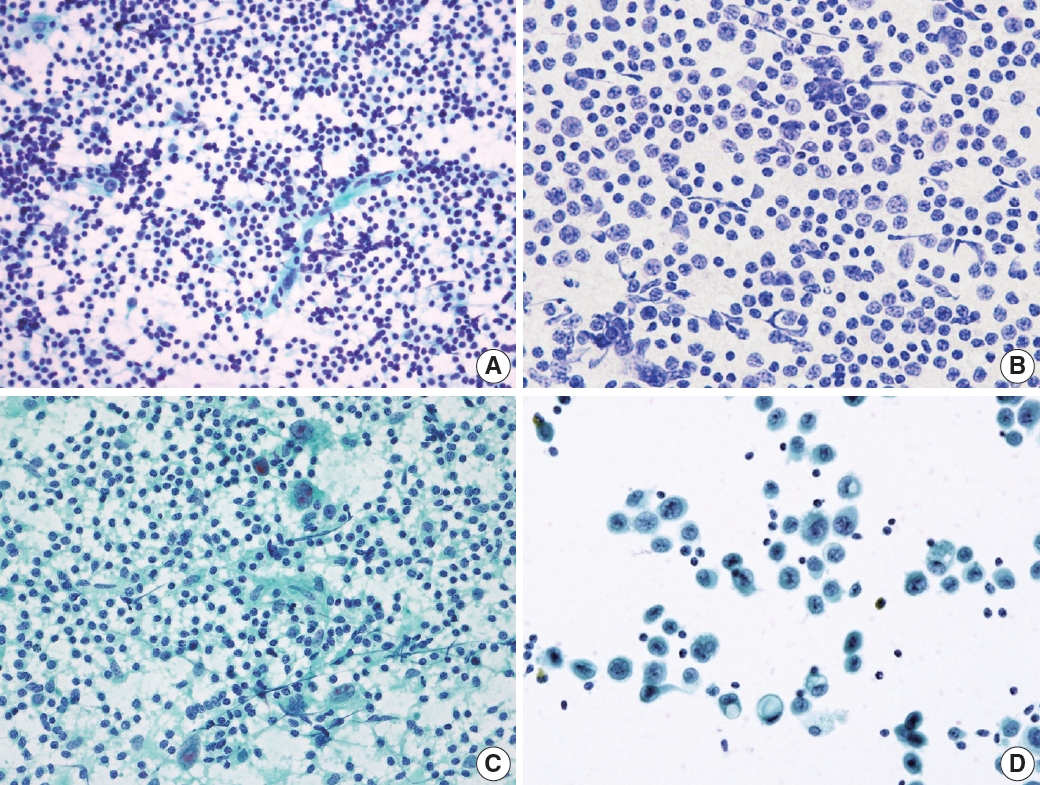
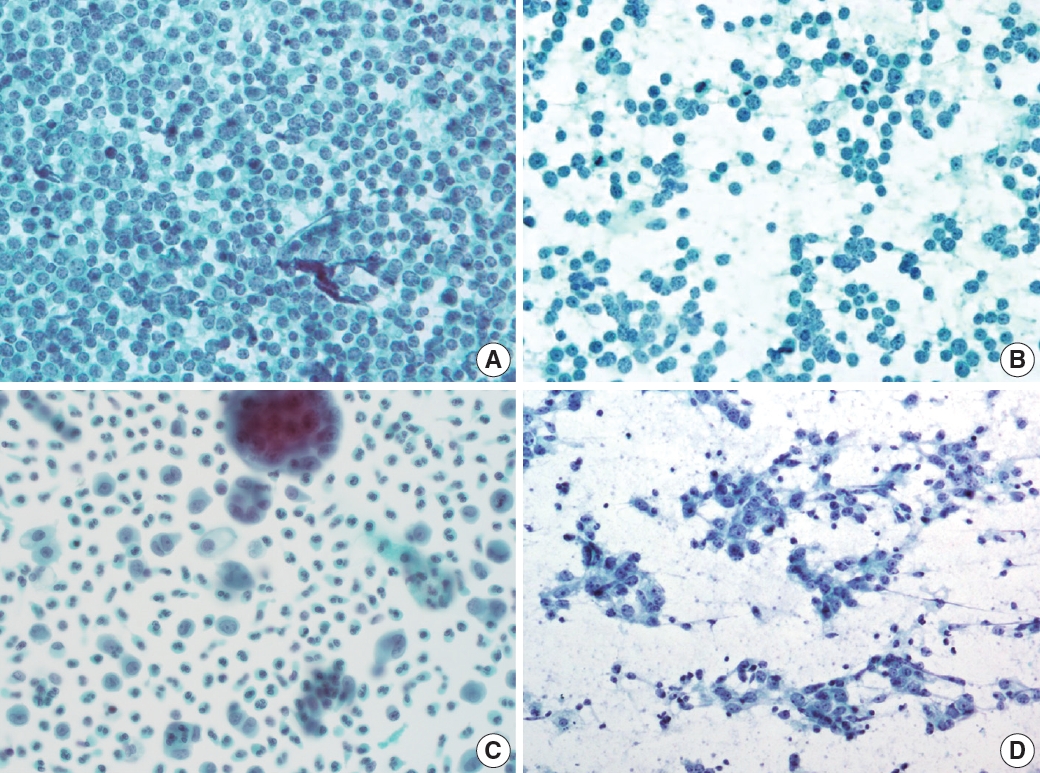
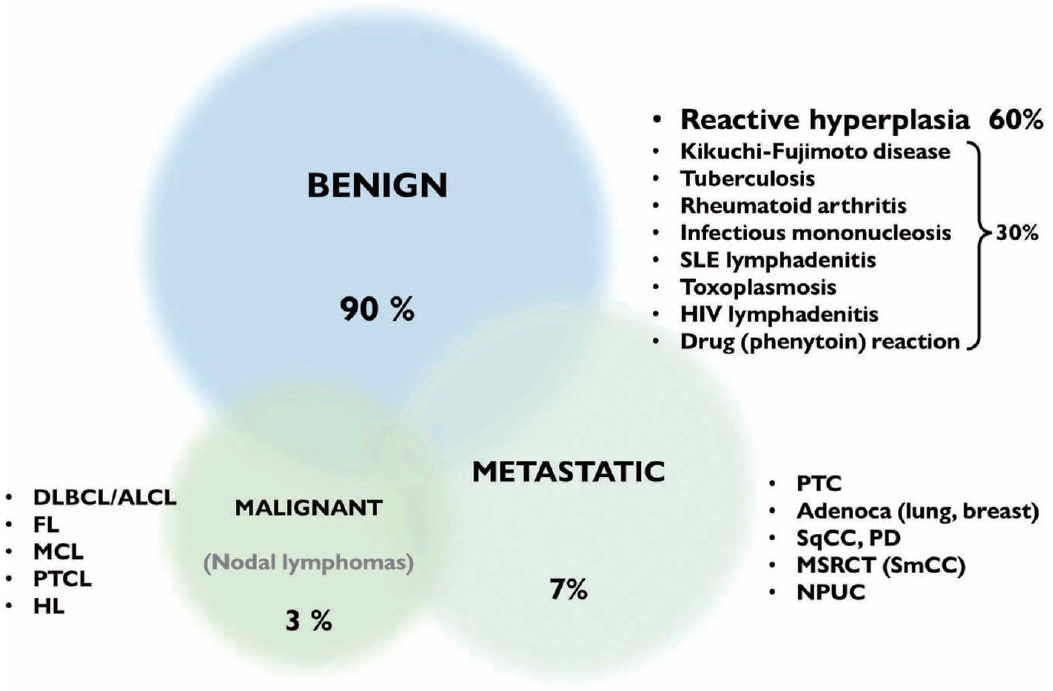
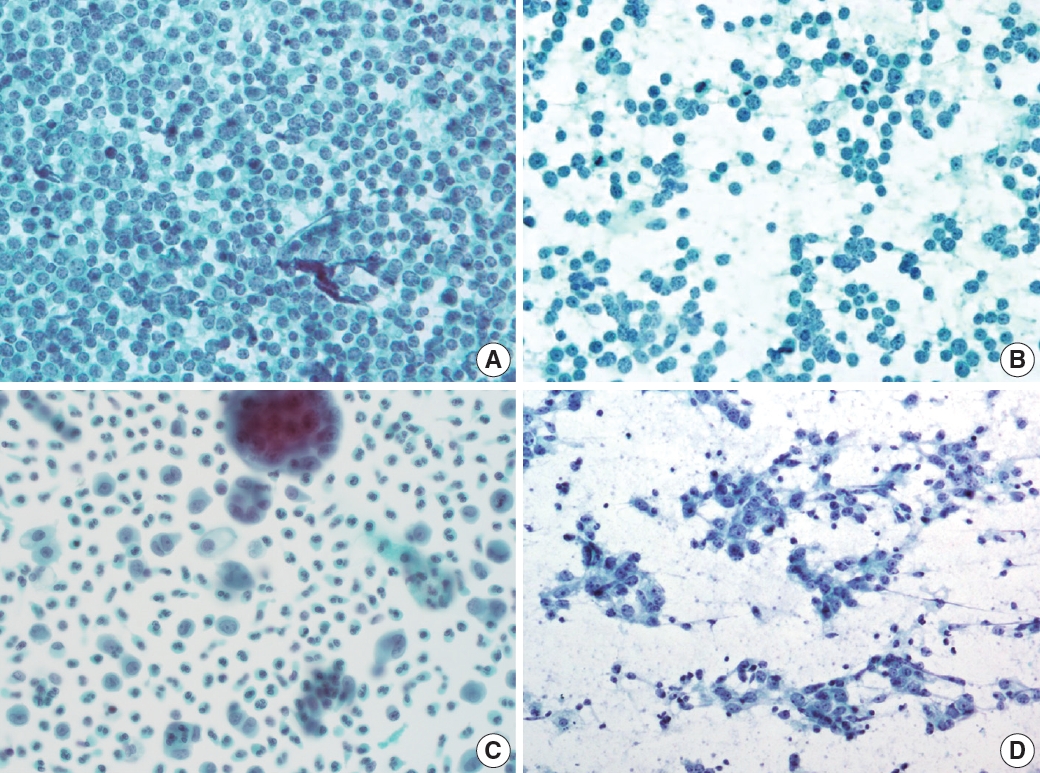
- Reactive lymphoid hyperplasia without a specific etiology accounts for 60% of all cases and usually demonstrates polymorphic cell populations, including small and large plasmacytoid lymphocytes [11]. This hyperplasia is often accompanied by tingible-body macrophages; dendritic lymphocytic aggregates (intact follicles); and other reactive inflammatory cells, including capillaries, eosinophils, and mast cells (Fig. 5A) [11].
- FL is another example of a two-cell population pattern. It demonstrates predominantly small irregular/cleaved and large cleaved/non-cleaved lymphocytes with few tingible-body macrophages, similar to reactive lymphoid hyperplasia (Fig. 5B). However, FL is more hypercellular, and the small lymphocytes are slightly larger than those in reactive lymphoid hyperplasia. Differential diagnosis can be challenging, although immunocytochemical staining for BCL2 may be helpful [12].
- HL also demonstrates two-cell populations comprising large Reed-Sternberg cells in the background of small lymphocytes, plasma cells, and histiocytes (Fig. 5C) [13]. Because the low-power cytologic findings of Hodgkin lymphoma may resemble those of reactive lymphoid hyperplasia, it is important not to miss Reed-Sternberg cells in high-power examinations.
- Rarely, differential diagnosis of metastatic carcinoma that demonstrates discohesive tumor cells with poor differentiation can be challenging as it can also present with two-cell populations, as in other examples (Fig. 5D). Tumor cells most often demonstrate adenoid or squamous differentiation, which can be useful for a correct diagnosis using high-magnification fields. Information on the clinical history or presentation of the primary cancer should be carefully verified when interpreting fine needle aspiration (FNA) slides.
- Continuous range of cell size population pattern
- A continuous range population pattern might be the most challenging pattern in lymph node FNA interpretation because it can be easily misinterpreted as reactive lymphoid hyperplasia. This pattern includes peripheral T-cell and angioimmunoblastic lymphoma, which are present in the background of diverse inflammatory cells including histiocytes, plasma cells, and eosinophils.
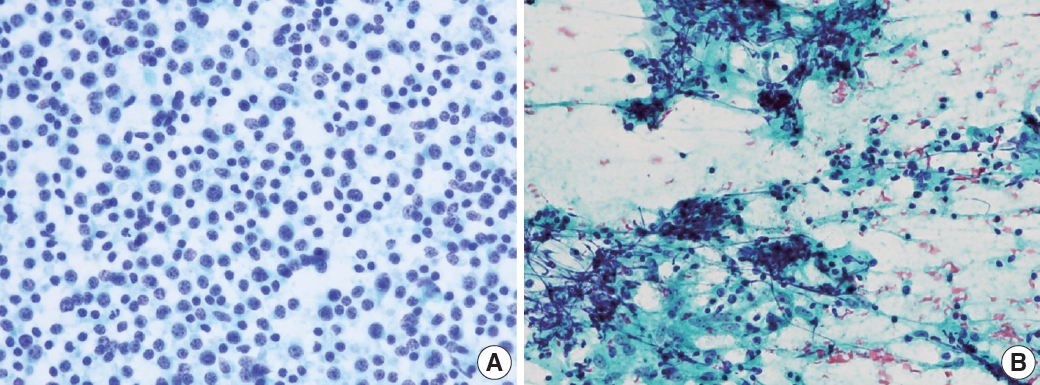
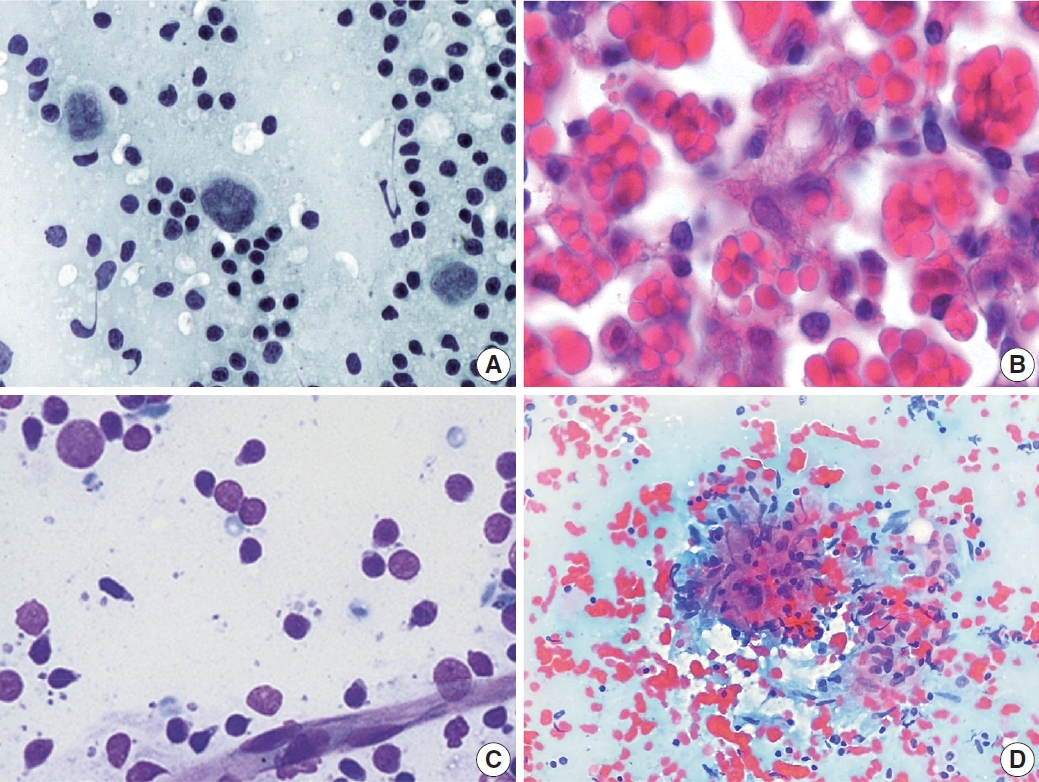
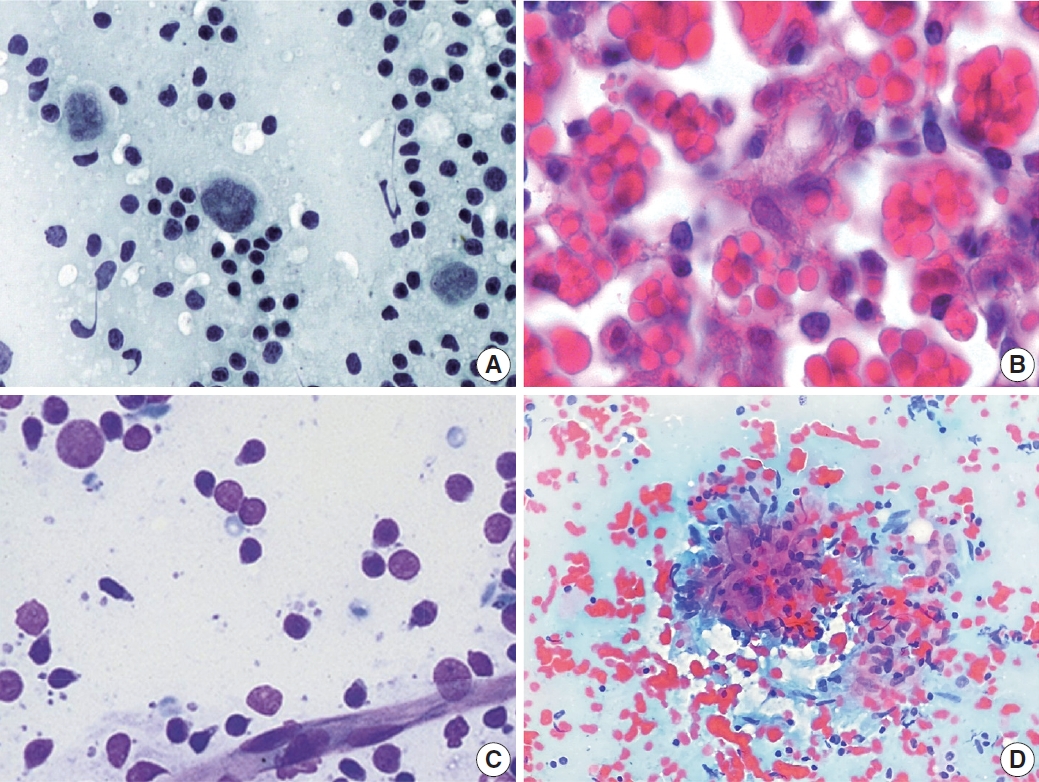
- PTCL demonstrated a marked variation in cell composition, including small, intermediate, and large cells (Fig. 6A) [14]. The biggest obstacle for proper diagnosis of PTCL is its lack of specific diagnostic features and immunocytochemical markers [15-17]. Likewise, there were no cytomorphological features specific to angioimmunoblastic T-cell lymphoma that displayed polymorphous cytomorphology (Fig. 6B). Sometimes, large cells resemble Reed-Sternberg cells and can be Epstein-Barr virus–positive, which leads to a misdiagnosis of HL.
- Monotonous small cell population pattern
- The third pattern involves a population of monotonously small cells and includes malignant small round cell tumors such as metastatic small cell carcinoma, alveolar rhabdomyosarcoma, and mature B-cell lymphomas (including mantle cell, lymphoblastic, and small lymphocytic lymphoma or chronic lymphocytic leukemia).
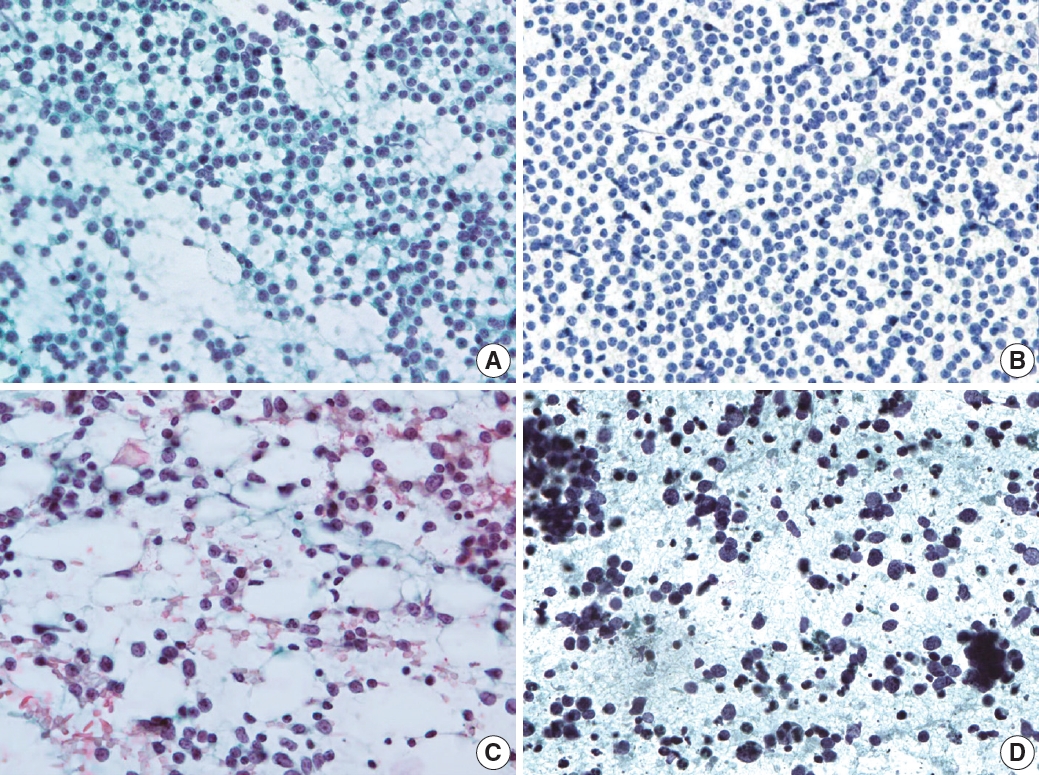
- Small lymphocytic lymphoma or chronic lymphocytic leukemia demonstrated monomorphous small lymphocytes with scanty cytoplasm, smooth or minimally irregular nuclei with clumped (soccer ball-like) chromatin, and inconspicuous or absent nucleoli (Fig. 7A) [18]. Occasionally, large lymphocytes such as prolymphocytes and paraimmunoblasts are observed. MCL demonstrated monomorphous small-to-intermediate-sized cells with scanty cytoplasm and irregular nuclei with fine chromatin and inconspicuous or absent nucleoli (Fig. 7B). Centroblasts or immunoblasts are absent. There may be a few histiocytes with moderately abundant eosinophilic cytoplasm, or socalled “pink histiocytes.” Lymphoblastic lymphoma demonstrates monomorphous intermediate-sized cells with scant or moderate cytoplasm and round or convoluted nuclei with finely granular chromatin and inconspicuous nucleoli [19]. The cells look small at low power due to their blastic nature. However, they are 1.5 to 2 times larger than the small lymphocyte at high power. Occasionally, cytoplasmic vacuoles and mitoses are observed (Fig. 7C).
- The most common diagnostic pitfalls in this category are malignant small round cell tumors, such as metastatic small cell carcinoma and rarely alveolar rhabdomyosarcoma. Small cell lung carcinoma commonly metastasizes to the cervical lymph nodes and demonstrates diverse cellular clusters and dispersed cells with abundant necrosis (Fig. 7D). Several important differential findings include variable-sized tumor cells with characteristic salt-and pepper-like chromatin including frequent smudging and molding. The differential interpretation of cell aggregation and clusters is important. Rarely, alveolar rhabdomyosarcoma can simulate the cytological features of malignant lymphomas. Therefore, the clinical presentation and history are important to avoid misdiagnosis.
- Monotonous large cell population pattern
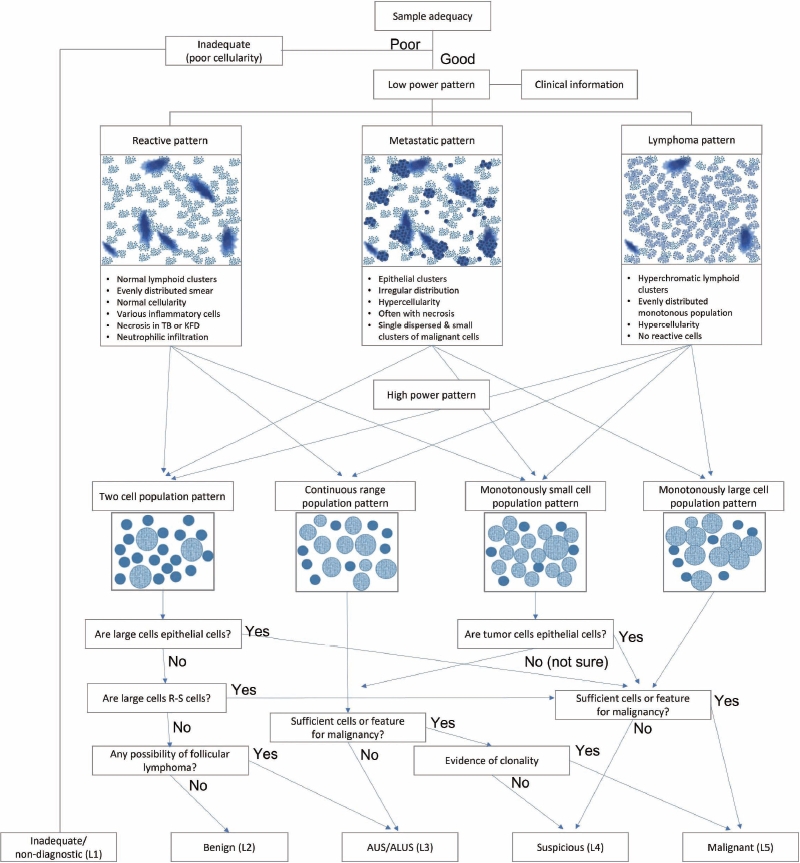
- The last pattern is a monotonously large cell population pattern. This pattern can be seen in high-grade lymphomas, such as diffuse large B-cell and Burkitt lymphoma, and other metastatic carcinomas, such as poorly differentiated squamous cell and nasopharyngeal undifferentiated carcinoma. The smear of diffuse large B-cell lymphoma demonstrated a moderately to highly cellular smear of predominantly large, atypical cells that were 2.5–5 times larger than small lymphocytes or histiocytes (Fig. 8A) [20]. The tumor cell has a round or irregular nucleus with a single prominent nucleolus and scant or abundant cytoplasm. However, these cells sometimes have multiple abnormal pleomorphic nuclei that resemble those of Reed-Sternberg cells. As reactive T-cells and histiocytes are few, they usually demonstrate a monotonously large cell population. Tingible-body macrophages are variable in number, lymphoglandular bodies are common, while dendritic lymphocytic aggregates are rarely seen.
- Burkitt lymphoma is another example of this pattern when the lesion involves extranodal sites. The tumor cells were typically uniform with intermediate-sized round nuclei, coarse chromatin, two–five small nucleoli per nucleus, and scant blue cytoplasm with small intracytoplasmic vacuoles (Fig. 8B). Several characteristic features of Burkitt lymphoma include apoptosis and brisk mitoses, along with frequent tingible-body macrophages.
- In this pattern, the major challenging differential diagnoses were poorly differentiated metastatic squamous cell and undifferentiated nasopharyngeal carcinomas that predominantly demonstrated singly dispersed tumor cells (Fig. 8C, D). It is important to identify tumor cells in clusters, which are uncommon in lymphoma.
HIGH-POWER PATTERN OF LYMPH NODE FINE NEEDLE ASPIRATION CYTOLOGY
- In addition to the low- and high-magnification power patterns, several characteristic findings are specific or pathognomonic for certain diseases (Fig. 2). One famous example is the occasional presence of Reed-Sternberg cells in Hodgkin lymphoma, which are large, atypical cells with multiple prominent nuclei and nucleoli intermixed with diverse inflammatory cells (Fig. 9A). Another example is the Russell body or Mott cells that can be found in mature B-cell lymphomas, including lymphoplasmacytic or extranodal marginal zone lymphoma (Fig. 9B) [21]. These findings are critical for proper diagnostic use of FNA samples. Therefore, it is important to be familiar with these disease-specific diagnostic clues. Lymphograndular bodies, also called hyaline bodies or lymphoid globules, are round, pale, basophilic fragments of cytoplasm with smooth borders in lymphoid tissues. Francis et al. [22] reported that lymphograndular bodies were found in >90% of non-Hodgkin lymphomas, 86% of reactive lymphadenitis, and 66% of Hodgkin lymphomas (Fig. 9C). Granulomas with caseous necrosis are suspicious for tuberculosis, while those that are small tight granuloma clusters without necrosis are suspicious for sarcoidosis (Fig. 9D). Granulomas can also be found in toxoplasmosis, cat-scratch disease, and HL. Other diagnostic clues include dendritic lymphocyte complexes in reactive hyperplasia, tingible-body macrophages in highly proliferative lymphomas such as Burkitt lymphoma, C-shaped macrophages in KFD, emperiopolesis in Rosai-Dorfman disease, and melanincontaining macrophages in dermatopathic lymphadenopathy. Additionally, Dutcher bodies are intranuclear inclusions of cytoplasm (pseudoinclusions) found in plasma cells and were originally described in Waldenstrom macroglobulinemia and IgA multiple myeloma. Arborizing vessels (high endothelial venules) can be found in the lymph node FNA of follicular dendritic cell-derived tumors, such as Castleman disease. A necrotic background is a common finding in several high-grade malignant lymphomas and metastatic carcinomas including diffuse large B-cell lymphomas or metastatic squamous cell carcinomas and in benign lymphadenopathies such as tuberculosis and necrotizing histiocytic lymphadenitis (KFD) [23].
DISEASE-SPECIFIC DIAGNOSTIC CLUES
- In 2019, a steering committee of international cytopathologists involved in lymph node FNAC developed a system for reporting lymph node FNAC in the International Cytology Congress in Sydney. They ultimately published The Sydney system after five rounds of circulation among committee members based on a review of the international literature and the expertise of the members (Table 1).
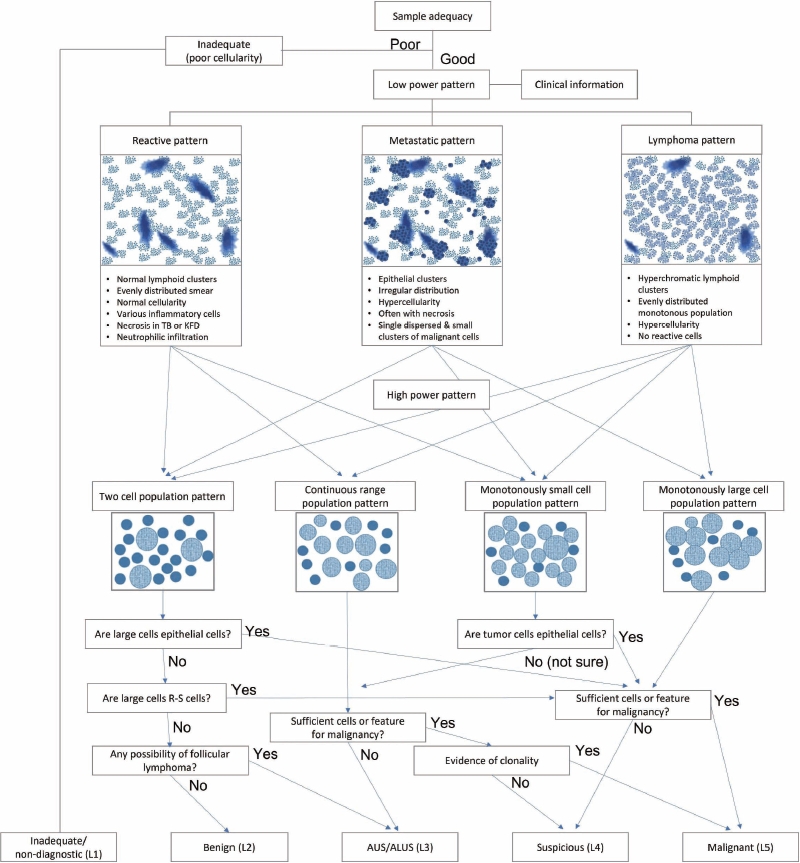
- This system defined the following five diagnostic reporting categories according to the cytological findings: inadequate/nondiagnostic (L1), benign (L2), atypical, undermined significance/ atypical lymphoid uncertain significance (AUS/ALUS) (L3), suspicious (L4), and malignant (L5) [2]. The inadequate/non-diagnostic category includes cases that cannot be diagnosed properly owing to scant cellularity, extensive necrosis, or technical limitations that cannot be overcome. Repeat FNAC, core needle, or excision biopsy was recommended in these cases. The benign category includes cases of suppurative and granulomatous inflammation and specific infections that demonstrate a heterogeneous lymphoid population (two-cell population). The AUS/ALUS category includes cases with two-cell populations in which the features suggest a reactive process; however, FL cannot be excluded; or the atypical cells are not lymphoid cells (AUS); or there is a monotonously small cell population for which lowgrade B-cell lymphomas cannot be excluded. The suspicious category includes cases with small and/or medium-sized monomorphic atypical lymphoid cells that are suspicious of lymphoma, but cytomorphology alone is insufficient to make the diagnosis. The suspicious category includes polymorphous lymphoid smears containing a few Reed-Sternberg-like cells, large cell or Burkitt lymphomas with scantly cellular cells, or atypical cells that are suspicious for metastasis are detected but are too scant to be diagnosed. The malignant category includes small- to mediumsized cells of non-HLs supported by evidence of clonality and all cases in which cytopathological features alone are sufficient to identify large cell non-HLs. This category also includes HL in cases in which there is an appropriate cellular background, diagnostic Reed-Sternberg cells, and metastatic neoplasms.
- The authors of the Sydney system proposed that this standardized system may improve the quality of the procedure, the handling of material for diagnostic ancillary testing, the understanding of the report, and the communication between the cytopathologist and the clinician. Recently, an Indian research group evaluated the malignancy risk in 6,983 lymph node FNACs by retrospectively reviewing cases with the Sydney system [24]. The diagnoses using the Sydney system were discordant in 10.7% of histologic diagnoses. The overall diagnostic accuracy of this system was 89.3%. The malignancy risk was 11.5% and 99.6% for the benign and malignant categories, respectively. Inter- and intraobserver variabilities were noted for categories 3 and 4, respectively. The authors reported a relatively high malignancy risk of 27.5% in the inadequate/non-diagnostic categories. For these cases, they recommended a repeat FNAC according to the Sydney system recommendations. Collectively, the authors concluded that application of the Sydney system can help achieve uniformity, reproducibility, and risk stratification in lymph node FNAC. However, multicenter studies with larger samples are needed to validate the utility of the Sydney system.
SYDNEY CLASSIFICATION
- Herein, we suggest a diagnostic algorithm for lymph node FNAC that encompasses a stepwise approach and the Sydney classification system (Fig. 10). Sample adequacy should be evaluated; if there is insufficient cellularity for proper evaluation or the smearing condition is poor due to dry artifacts, then the sample can be categorized as inadequate/non-diagnostic (L1). Once the clinical information is reviewed, the low-power pattern can be evaluated under a microscope. Reactive patterns can present as two-cell populations, a continuous range, or a monotonously small cell population. Metastatic patterns can present as two-cell populations of monotonously small or large cell populations. Lymphoma can present with all kinds of patterns at high-power magnification. A large cell population in the two-cell population pattern should be thoroughly reviewed if it has epithelial features to exclude the possibility of a metastatic lesion. If there is no chance of metastasis, FL remains a possibility. FL should be considered in cases of multiple enlarged lymph nodes in elderly patients with hypercellular smears and several small lymphocytes that are slightly larger than usual. Immunocytochemical staining for BCL2 can be helpful for diagnosis in such cases. When FL cannot be excluded and immunocytochemical staining is unavailable, an ALUS diagnosis can be made.
- If there are large cells are clearly epithelial, and cytologic atypia is evident, the case can be diagnosed as malignant or suspicious according to the level of evidence. This is true regardless of the high-power pattern of a two-cell, monotonously small, or large cell population. A monotonously large cell population is mostly found in high-grade B-cell lymphomas, such as diffuse large B-cell or Burkitt lymphoma; however, such cases can also be diagnosed as malignant or suspicious according to the level of evidence. Monotonously small cell population patterns can be quite challenging because certain features can be difficult to distinguish between lymphomas and small cell carcinoma (such as mild cytologic atypia in lymphoma). If there are enough cytological features for small cell carcinoma, a case can be diagnosed as malignant or suspicious according to the level of evidence. If there is no definite evidence that the tumor cells are epithelial, lymphomas or other rare mesenchymal malignant tumors should be excluded. In such cases, cytological findings and additional IHC or molecular studies might be required. These cases can be diagnosed as AUS/ALUS, suspicious, or malignant according to the level of evidence. The continuous range population pattern is predominantly from possible T-cell lymphomas. Clonality tests, such as TCR gene rearrangement, are required for a more conclusive diagnosis.
- The Sydney system has an inevitable limitation in that the epithelial or lymphomatous nature of the lesion is not clearly separated into different categories and is not emphasized due to the ambiguity of the lymph node features on FNAC. In other words, even though a diagnosis was made on a certain case using the Sydney system, it is still unclear whether the case is lymphoid or metastatic. The L3 category, which includes AUS and ALUS, is a good example. It is often quite challenging to discriminate a certain lesion as lymphoid or epithelial in origin. The aforementioned study by the Indian group mentioned intra- and interobserver variabilities in the diagnosis of category III. However, further in-depth knowledge of lesion nature might be warranted. In cases in which the FNAC features favor certain diseases, the cases should be described specifically as “AUS, favor poorly differentiated carcinoma,” “ALUS, favor atypical lymphoproliferative disease (Infectious mononucleosis, Kikuchi-Fujimoto disease, Rosai-Dorfman disease),” or “ALUS, cannot exclude low-grade lymphoma.” Since the previous study reported a high malignancy rate of 66.7% in the AUS/ALUS category, it is important to deliver any evidence that supports either lymphoid or metastatic lesion. However, it is also important not to conduct unnecessary excisional biopsy.
SUGGESTED DIAGNOSTIC ALGORITHM FOR LYMPH NODE FINE NEEDLE ASPIRATION CYTOLOGY
- Lymph node FNAC encompasses diverse diseases, including benign and malignant lymphoma and metastasis, whose cytologic findings often resemble each other. A stepwise diagnostic approach combining clinical findings (age, sex, site, multiplicity, ultrasonography findings), low-power pattern (reactive, metastatic, lymphoma pattern), high-power population pattern (two-cell, continuous range, monotonously small, and monotonously large population patterns), and disease-specific diagnostic clues (granulomas, etc.) can help in comprehensive FNAC diagnosis. It is important to remember representative traits of each diagnostic category, including diagnostic pitfalls that share the cytologic findings of other categories.
CONCLUSION
Supplementary Information
Supplementary Table S1.
Supplementary Fig. S1.
-
Ethics Statement
This study was reviewed and approved by the Institutional Review Board of the Catholic University of Korea College of Medicine (UC21ZISI0138).
-
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
-
Code Availability
Not applicable.
-
Author Contributions
Conceptualization: YC, SSL, CSK. Data curation: YC, JAG, CSK, SSL, HJC, HJK, GP. Funding acquisition: YC. Investigation: YC, SSL. Methodology: YC. Project administration: YC. Resources: YC, CSK, SSL, HJC, HJK, GP. Supervision: SSL, GP, CSK. Validation: YC, JAG. Visualization: YC, JAG. Writing—original draft: YC, JAG. Writing—review & editing: YC, SSL, HJC, HJK, CSK, GP. Approval of final manuscript: all authors.
-
Conflicts of Interest
Y.C., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
-
Funding Statement
This study was supported by a Korean Society for Cytopathology grant (No. 2021-01).
Notes

- 1. Zhou J, Li F, Meng L, et al. Fine needle aspiration cytology for lymph nodes: a three-year study. Br J Biomed Sci 2016; 73: 28–31. ArticlePubMed
- 2. Cibas ES, Ducatman BS. Cytology: diagnostic principles and clinical correlates. 5th ed. Maryland Heights: Elsevier Inc, 2020.
- 3. Rammeh S, Romdhane E, Sassi A, et al. Accuracy of fine-needle aspiration cytology of head and neck masses. Diagn Cytopathol 2019; 47: 394–9. ArticlePubMedPDF
- 4. Houcine Y, Romdhane E, Blel A, et al. Evaluation of fine needle aspiration cytology in the diagnosis of cervical lymph node lymphomas. J Craniomaxillofac Surg 2018; 46: 1117–20. ArticlePubMed
- 5. Rammeh S, Ben Rejeb H, M’Farrej M K, et al. Cervical node fine needle aspiration: factors influencing the failure rate. Rev Stomatol Chir Maxillofac Chir Orale 2014; 115: 85–7. PubMed
- 6. Heo I, Park S, Jung CW, et al. Fine needle aspiration cytology of parathyroid lesions. Korean J Pathol 2013; 47: 466–71. ArticlePubMedPMC
- 7. Agarwal AM, Bentz JS, Hungerford R, Abraham D. Parathyroid fine-needle aspiration cytology in the evaluation of parathyroid adenoma: cytologic findings from 53 patients. Diagn Cytopathol 2009; 37: 407–10. ArticlePubMed
- 8. Gronkiewicz JJ, Vade A. Cervical lymph node fine needle aspiration in patients with no history of malignancy. Ultrasound Q 2013; 29: 323–6. ArticlePubMed
- 9. Hong SA, Jung H, Kim SS, et al. Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice. J Pathol Transl Med 2022; 56: 361–9. ArticlePubMedPMCPDF
- 10. Al-Abbadi MA, Barroca H, Bode-Lesniewska B, et al. A proposal for the performance, classification, and reporting of lymph node fine-needle aspiration cytopathology: the Sydney system. Acta Cytol 2020; 64: 306–22. ArticlePubMedPDF
- 11. Duraiswami R, Margam S, Chandran P, Prakash A. Spectrum of pathologies on FNAC evaluation of peripheral lymph nodes at a tertiary care center in hyderabad: a retrospective study. Int J Adv Med 2017; 4: 27–33. Article
- 12. Cho J. Basic immunohistochemistry for lymphoma diagnosis. Blood Res 2022; 57: 55–61. ArticlePubMedPMC
- 13. Kuppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol 2005; 37: 511–7. ArticlePubMed
- 14. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol 2013; 26 Suppl 1: S71–87. ArticlePubMedPDF
- 15. de Leval L, Savilo E, Longtine J, Ferry JA, Harris NL. Peripheral Tcell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am J Surg Pathol 2001; 25: 395–400. ArticlePubMed
- 16. Huang Y, Moreau A, Dupuis J, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol 2009; 33: 682–90. ArticlePubMedPMC
- 17. Dobay MP, Lemonnier F, Missiaglia E, et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica 2017; 102: e148–51. ArticlePubMedPMC
- 18. Rizzo K, Nassiri M. Diagnostic workup of small B cell lymphomas: a laboratory perspective. Lymphoma 2012; 2012: 346084.ArticlePDF
- 19. Jacobs JC, Katz RL, Shabb N, el-Naggar A, Ordonez NG, Pugh W. Fine needle aspiration of lymphoblastic lymphoma: a multiparameter diagnostic approach. Acta Cytol 1992; 36: 887–94. PubMed
- 20. Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ. Diffuse large Bcell lymphoma variants: an update. Pathology 2020; 52: 53–67. ArticlePubMed
- 21. el-Okda M, Hyeh Y, Xie SS, Hsu SM. Russell bodies consist of heterogenous glycoproteins in B-cell lymphoma cells. Am J Clin Pathol 1992; 97: 866–71. PubMed
- 22. Francis IM, Das DK, al-Rubah NA, Gupta SK. Lymphoglandular bodies in lymphoid lesions and non-lymphoid round cell tumours: a quantitative assessment. Diagn Cytopathol 1994; 11: 23–7. ArticlePubMed
- 23. Jimenez-Heffernan JA, Diaz Del Arco C, Adrados M. A cytological review of follicular dendritic cell-derived tumors with emphasis on follicular dendritic cell sarcoma and unicentric Castleman disease. Diagnostics (Basel) 2022; 12: 406.ArticlePubMedPMC
- 24. Gupta P, Gupta N, Kumar P, et al. Assessment of risk of malignancy by application of the proposed Sydney system for classification and reporting lymph node cytopathology. Cancer Cytopathol 2021; 129: 701–18. ArticlePubMedPDF
References
Figure & Data
References
Citations

- Immunocytochemical markers, molecular testing and digital cytopathology for aspiration cytology of metastatic breast carcinoma
Joshua J. X. Li, Gary M. Tse
Cytopathology.2024; 35(2): 218. CrossRef - Response to comment on “A stepwise approach to fine needle aspiration cytology of lymph nodes”
Yosep Chong, Gyeongsin Park, Hee Jeong Cha, Hyun-Jung Kim, Chang Suk Kang, Jamshid Abdul-Ghafar, Seung-Sook Lee
Journal of Pathology and Translational Medicine.2024; 58(1): 43. CrossRef - Comment on “A stepwise approach to fine needle aspiration cytology of lymph nodes”
Elisabetta Maffei, Valeria Ciliberti, Pio Zeppa, Alessandro Caputo
Journal of Pathology and Translational Medicine.2024; 58(1): 40. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission